| Model | : | WQS485ORP | |
| Maximum Pressure | : | 50Psig | |
| Communication | : | RS485 Modbus | |
| IP Rating | : | IP68 | |
| Power Input | : | DC12V | |
| Measurement Method | : | Glass Bulb | |
| Parameter | : | ORP | Temperature |
| Range | : | -1500 to 1500mV | 0 to 50oC |
| Accuracy | : | ±5mV | ±0.2 |
| Resolution | : | 1 | 0.1 |
WQS485ORP – ORP
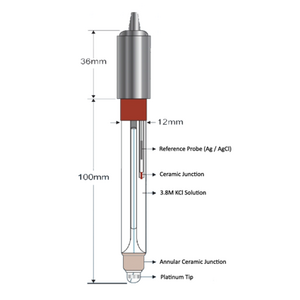
WQS485ORP is a digital output water ORP sensor developed and manufactured by MJ Instruments.
With the sensor body filled internally which acts as sensor weights as well as protecting the electronic components, the sensor is well suited for long-term deployment.
The body is built with corrosive-resistant material, allowing it to be deployed at industrial discharge for pollution monitoring.
Different housing is also available upon request. With standard RS485 Modbus output, it is easy to integrate the sensor into the commercial controllers.
| ORP Range | -1000 to 1000mV |
|---|---|
| ORP Accuracy | +-5 |
| ORP Resolution | 1 |
| Temperature Range | 0 – 50 Deg C |
| Temperature Accuracy | +- 0.2 |
| Temperature Resolution | 0.1 |
| Max Pressure | 50 Psig |
| Reference Solution | Ag/AgCl Double Salt Bridge |
| Communication | RS485 Modbus |
| Protection Rating | IP68 |
| Power Supply | DC 12V |